The audit room was tense and with pile up of SOPs. A stack of batch records sat between the QA lead and the auditor, and everyone knew there was a problem in one of them. Not a catastrophic failure — but a small deviation in SOP that had never been reported.
If you’ve been in GMP long enough, you’ve seen this story before. Someone skips a step of SOP, follows “how we usually do it” instead of what’s written in the SOP, or makes a “temporary” change without approval. Later, during an inspection, that “small thing” becomes the opening line of a 483 observation or an inspection finding.
Why does this happen in environments where compliance is drilled into us? And more importantly, how do we fix it — without burning out our teams or making SOPs into binders nobody reads?
In this article, we’ll explore the psychology of why SOPs aren’t always followed, the human factors behind it, and practical ways to rebuild SOP ownership across your teams.
Why SOP Exist — And Why People Still Skip Steps
We all know the textbook reason: SOPs ensure consistency, compliance, and safety. But in practice? Many employees view them as bureaucratic paperwork that slows them down.
I once walked into a cleanroom where a new operator had been trained on the correct gowning procedure.
Yet, during the shift, they skipped the second pair of gloves.
Why? “It’s faster this way — and everyone does it like this when no one’s watching.”
This isn’t about laziness.
It’s about perception.
If the operator’s daily reality tells them speed is valued over strict compliance, they’ll unconsciously align their behavior with what the culture rewards.
Here are the 10 Reasons Why People Don’t Own SOPs.You can also explore my in-depth LinkedIn newsletter on this topic for more real-world GMP insights.

The Early Warning Signs You’re Slipping Into SOPs Non-Compliance
Non-compliance doesn’t start with a critical deviation.
It begins with small behaviors that quietly erode GMP discipline.
You might notice:
-
Operators writing from memory instead of following instructions step-by-step.
-
Minor undocumented changes to process timing.
-
Batch record entries made “later” instead of in real time.
-
Workarounds for inconvenient controls.
Each one seems harmless until you zoom out.
What’s dangerous is not the act itself, but the normalization of deviation.
The worst part? By the time these habits surface in an audit, the behaviors are already deeply embedded in the culture.
Here are the – 11 Early Warning Signs in GMP Environments.

Read the full FDA 21 CFR Part 211 regulation here.
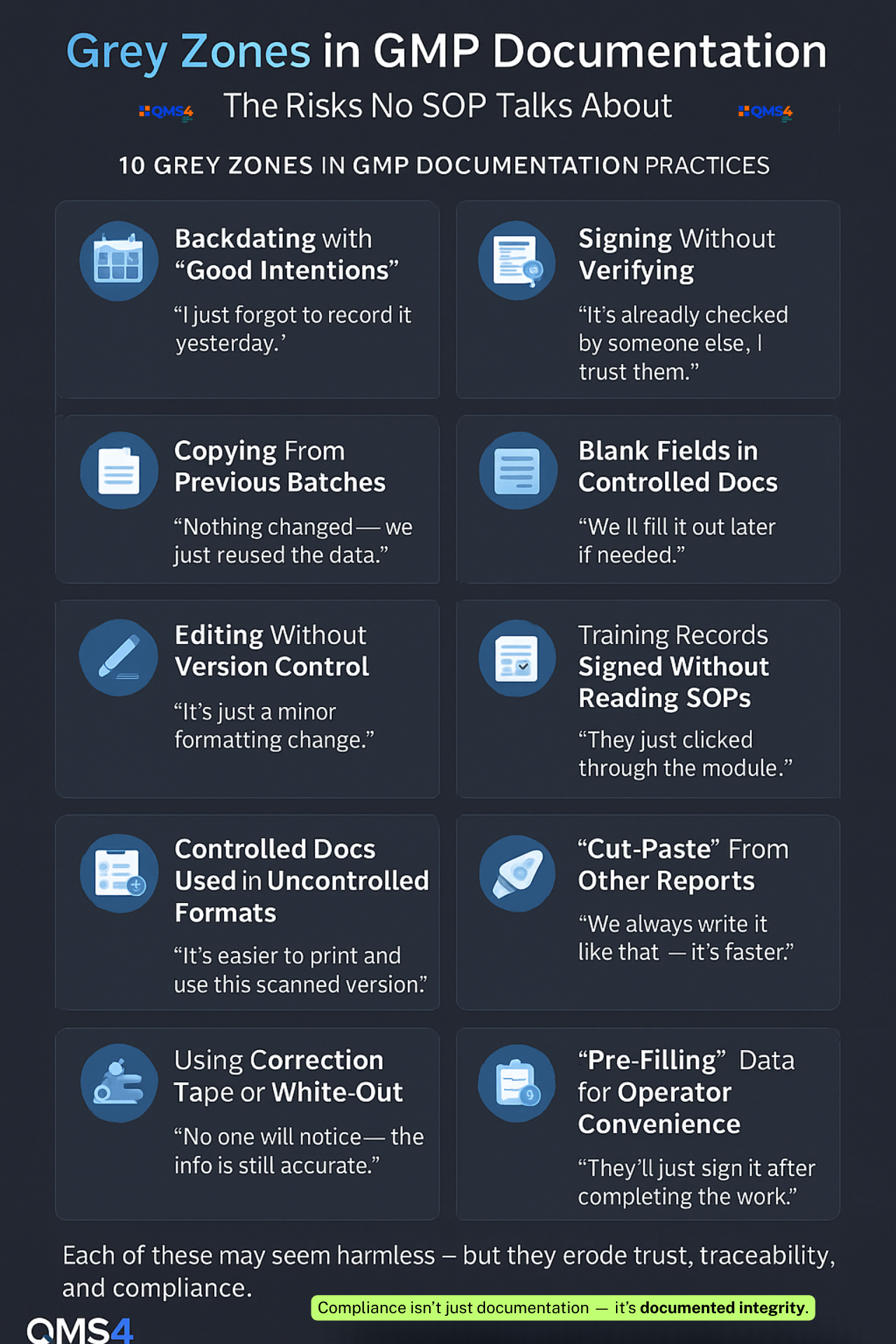
The Grey Zones That Invite SOP Shortcuts
Every facility has them — the “grey zones” where procedures aren’t crystal clear, or where the wording leaves room for interpretation.
These gaps in documentation create a breeding ground for “personal versions” of the SOP.
Example:
An SOP says, “Visually inspect the equipment for cleanliness before use.”
No one defines what “cleanliness” means, or documents the inspection step with photos or checklists.
So, each operator decides what’s “clean enough.”
From a QA perspective, this is a nightmare.
From an operator’s perspective, it’s just making a judgment call in the moment.
Here are the – 10 Grey Zones in GMP Documentation
Read the PIC/S Guide to GMP for detailed global quality requirements.
The SOP Compliance Struggles Nobody Talks About
In most GMP shops, there’s a silent tension:
Production feels pressured to meet output targets.
QA feels pressured to ensure compliance no matter the schedule.
This tension shows up as:
-
Frustration when QA rejects work.
-
Resistance to procedural changes.
-
Operators feeling QA “slows everything down.”
The truth is, SOP ownership is low when teams feel SOPs are designed for auditors instead of for the people doing the work.

Read the WHO GMP Guidelines to understand internationally recognized GMP standards.
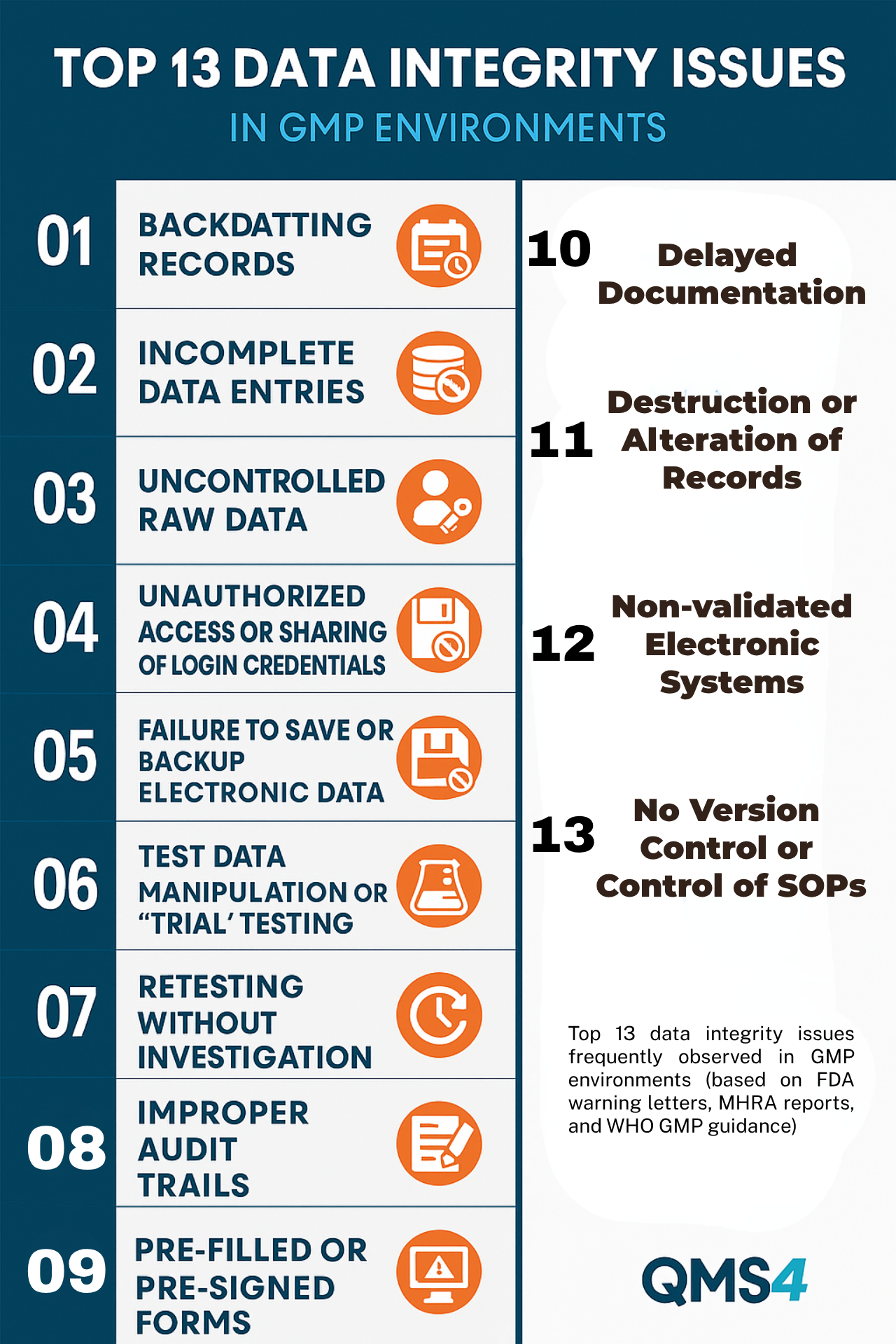
Data Integrity — Where Small Slips in SOP Become Major Findings
Data integrity issues are rarely the result of malicious intent.
Most often, they start with small, pressured decisions:
“I’ll fill this in later.”
“This test result is obviously okay — I don’t need to double-check.”
Over time, these shortcuts form habits, and habits leave trails in your audit logs.
Whether it’s missing signatures, out-of-order entries, or undocumented corrections — each one can trigger a regulatory citation.
Here are : Top 13 Data Integrity Issues
Read the MHRA GxP Data Integrity Guidance for UK regulatory expectations.
How to Increase SOP Ownership Across Your Teams
Improving SOP compliance isn’t just about retraining.
It’s about changing how people perceive and value the SOP itself.
Some practical approaches:
-
Involve operators in SOP drafting.
-
Use visual SOPs where possible.
-
Align KPIs so compliance is rewarded alongside productivity.
-
Encourage feedback loops for SOP improvements.
-
Make SOPs easy to navigate on the shop floor.
Here are: Top 8 Ways to Increase SOP Ownership

Read the ICH Q10 Pharmaceutical Quality System guideline for a structured approach to GMP compliance.
Reflective Takeaway
GMP isn’t just about doing things right.
It’s about building systems where people want to do things right — because they see the value, not just the rule.
When SOP ownership is high, audits stop being fear-driven events.
They become proof points of a healthy quality culture.
FAQs – SOP Compliance
-
Why do people often skip following SOPs in GMP environments?
Many employees face time pressure, unclear instructions, or lack motivation, causing them to bypass SOP steps despite knowing their importance. -
Is skipping SOPs always due to human error?
Not necessarily. Often, it reflects systemic issues like poorly designed procedures, inadequate training, or a culture that discourages reporting deviations. -
How can quality leaders reduce SOP non-compliance?
Leaders can model good behavior, foster open communication, and involve teams in creating realistic, user-friendly SOPs. -
What role does workplace culture play in SOP adherence?
A positive culture where employees feel safe to speak up encourages consistent SOP compliance and reduces hidden deviations. -
How does burnout impact SOP compliance?
Burnout reduces focus and motivation, making employees more prone to take shortcuts or overlook critical steps. -
Can behavioral CAPA improve SOP adherence?
Yes, by addressing root causes beyond retraining, such as mindset, environment, and leadership support, behavioral CAPA leads to sustainable improvements. -
What practical steps can teams take to make SOPs more followable?
Using visual aids, simplifying language, piloting SOPs with operators, and soliciting regular feedback helps make SOPs easier to follow. -
How does audit readiness relate to SOP compliance?
Consistent SOP adherence minimizes deviations and findings during audits, ensuring smoother inspections and regulatory trust.
Want more insights like this?
Connect with me on LinkedIn for – “Quality Career & GMP Insights”.
Follow QMS4 | Connect with Lokman | Subscribe to Newsletter (Quality Career and GMP Insights) |
👇 Drop a comment, share your experience or DM me — your voice matters. It might help another quality professional break free from burnout. And honestly, it inspires me to keep writing….