Let’s start with a hard truth.
If Quality by Design (QbD) was meant to revolutionize pharma quality systems, why do we still spend 18 to 24 months preparing for a 3-day inspection? Why does the fear of a 483 still loom like a dark cloud over audit readiness?
Why do major findings still show up — even after all the procedures, protocols, validations, and controls are in place?
And here’s one that stings:
“We wrote 40 SOPs in three weeks — just for the audit. Not for GMP compliance. Not for sustained quality.”
Sound familiar?
If you’ve ever seen 30, 40, or even 50 SOPs signed on the same date, you’ve probably asked:
“When did they train people on all this?”
Most SOPs aren’t written to help people do the job better — they’re written to pass audits.
What’s Really Broken in Pharma QMS?
Let’s go deeper:
- Change control feels like bureaucracy, not improvement
- QMS software slows users down instead of supporting them
- Internal audit reports get filtered to avoid political tension
- We settle for symptoms instead of root causes — because closure, not learning, is the priority
- Deviation classifications get manipulated to avoid escalation
If you’ve been in quality long enough, none of this surprises you.
And yet — we rarely say it out loud.
Why This Article Matters
In quality, we’ve spent decades perfecting how to design quality into products. Traditional QbD was a breakthrough — it taught us to build quality into the process from the beginning, not inspect it at the end.
But after 20 years in the field — 17 of those inside global multinational companies — I’ve come to a humbling realization:
“We can design the perfect process on paper. But if people don’t think, act, or decide with quality in mind — it doesn’t matter.”
That’s where traditional QbD falls short.
So here’s the uncomfortable question:
What if QbD has a blind spot?
A blind spot so critical that it explains why even mature systems still buckle under pressure?
QbD, in its traditional form, focuses on product specs, process controls, and risk assessments.
But it misses something fundamental:
- How people behave under pressure
- How decisions are made in grey zones
- How culture shapes shortcuts, workarounds, and silence
In short: QbD designs quality into processes — not people.

When the Audit Alarm Bell Rings
It was 6:47 a.m. on a rainy Thursday when Rina, the QA Officer, got the message:
“Regulatory audit scheduled next week. Full scope. Be ready.”
Her stomach dropped.
The site had just wrapped a painful CAPA cycle.
The training tracker showed multiple overdue records.
The new QMS software still glitched on uploads.
And there were whispers — always whispers — of skipped verifications on night shifts.
Rina took a deep breath. Not because she didn’t know what to do.
But because she did.
- Fourteen-hour shifts
- “Urgent” document updates
- Backdated training
- Compliance firefighting
- People walking on eggshells
“We design quality into the product,” her manager would say.
But no one talked about the decisions made at 2 a.m. under pressure.
No one talked about designing quality into behavior — not just documents.
Why Good People Still Miss Things
This article is for every professional who has asked:
- Why do experienced, well-intentioned people still make mistakes in GMP settings?
- Why does compliance feel reactive instead of built-in?
- Why does traditional QbD stop at the product?
Let’s consider a typical deviation:
A critical step was missed during line clearance.
The investigation follows protocol:
Deviation logged. Fishbone diagram. 5 Whys. RCA template. CAPA logged and closed.
But we know the deeper story:
- The operator skipped the step because the batch record was confusing
- The line lead didn’t double-check because they were short-staffed
- The engineer didn’t escalate to avoid tension
- The supervisor gave verbal approval — “just this once”
Each of these actions is behavioral — not procedural.
Traditional QbD assumes that if systems are right, people will follow.
But people don’t always work that way.
Real Life Example: The QA Officer Who “Failed” for Doing the Right Thing
Let’s think of a scenerios, Olivia — a new QA officer — flagged a critical cleaning validation gap.
She was correct. She was committed.
But her feedback caused a batch delay.
The production head was annoyed.
The site director questioned her timing.
Her manager advised her to be more “practical.”
Three months later, her contract wasn’t renewed.
What message does that send?
You can design the SOP.
You can design the policy.
But if you don’t design for accountability, courage, and trust, the system punishes good decisions.
The Psychology of Quality: Systems, Nudges, and Biases
People don’t always make decisions based on logic.
We’re influenced by emotion, context, memory, fatigue, and social cues.
Even when QbD systems are technically sound, quality outcomes can be undermined by predictable human behavior:
Checklist Blindness
- Fields marked “critical” become background noise
- Red flags get ignored if they’ve never caused issues
- People stop “seeing” what they see every day
How to fix it:
- Reduce checklist fields by 20%
- Use icons and color cues
- Rotate formats periodically
CAPA Fatigue
- Multiple deviations = one generic “training” CAPA
- Root causes repeat: “human error,” “lack of attention”
- Closure becomes a checkbox, not a change
Truth: Human error is a symptom — not a cause.
If the system doesn’t change, neither will the outcome.
Cognitive Bias in Risk Assessments
- We assume others understand what we understand
- Familiarity lowers our risk perception
- We over-trust procedural compliance
These aren’t process flaws. They’re human perception gaps.
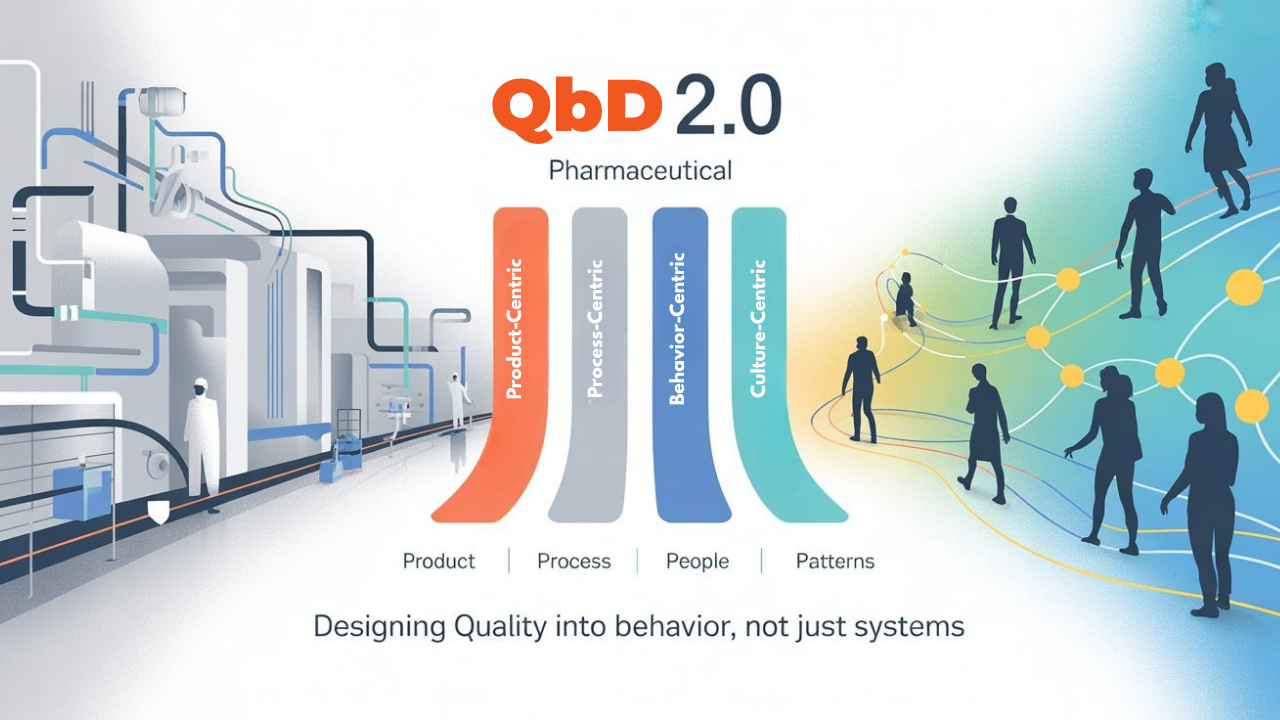
QbD 2.0 = Product + Process + People + Patterns
To evolve QbD, we must design not just for systems, but for behavior.
Here’s what QbD 2.0 looks like:
1. Product
Traditional: Define Critical Quality Attributes (CQAs)
QbD 2.0: Also define user risk behaviors (e.g., misuse, storage errors)
2. Process
Traditional: Control CPPs, ensure process capability
QbD 2.0: Also build behavioral friction (e.g., smart deviations, forcing functions)
3. People
Traditional: Train staff on SOPs
QbD 2.0: Also design culture cues (e.g., feedback loops, peer norms, psychological safety to speak)
4. Patterns
Traditional: Analyze audits, metrics, deviations
QbD 2.0: Also map behavioral drift over time (e.g., CAPA fatigue, normalization of shortcuts)
Where to Apply This Framework
Use QbD 2.0 principles in:
- RCA investigations
- Internal audits
- SOP and checklist design
- Onboarding programs
- Continuous improvement cycles
Ask not just: “Did they follow the SOP?”
Ask:
- “What made the wrong action easier?”
- “What do we tolerate that reinforces poor decisions?”
- “What makes the right choice harder than it should be?”
Design Quality into Decision Points — Not Just Deliverables
Every procedure is a decision environment.
- Will the analyst document honestly — or quietly “correct” a mistake?
- Will the technician stop when something feels off — or keep going?
- Will the QA reviewer raise a flag — or avoid tension?
Don’t just define the right step.
Design the environment that makes it easier to take.
Behavioral Design in Action
1. Smart Deviation Review
Add a field: “Was any workaround used, even if no deviation occurred?”
This normalizes discussion of behavioral gaps before they become audit findings.
2. Reinforcement Messaging
Instead of annual training on ALCOA+, display monthly micro-reminders on screensavers, passcards, or checklists:
“Integrity = I do it the way I say I do it.”
Simple, frequent repetition works better than dense training slides.
3. Pre-Mortem Before Process Changes
Before implementing a change, ask the team:
“What’s the most likely way this will be ignored or bypassed in 6 months?”
This activates real-world behavioral foresight — not just procedural theory.
Why This Matters: The Future of Quality Is Behavioral
By 2030, the most compliant sites won’t just have perfect documentation.
They’ll have:
- Teams that trust and challenge
- Cultures that reinforce integrity
- Systems that nudge the right choice
They will:
- Design SOPs that are usable, not just compliant
- Write deviations that tell stories, not tick boxes
- Create systems that help people want to do the right thing
This isn’t about being soft — it’s about being strategic.
Because systems don’t behave. People do.

Final Reflection: Quality is a Human System
Let’s go back to Olivia — the QA officer from the beginning.
What if her company didn’t just fix documents for the audit?
What if they fixed the behavioral gaps the system creates?
Imagine a workplace where:
- Supervisors don’t fear escalation
- Checklists are designed to engage
- Human error is investigated, not punished
- QbD includes behavior — not just specs and controls
“Every system is perfectly designed to get the results it gets.” — W. Edwards Deming
If we want better outcomes, we must build better systems — and that means better behavior by design.
Key Takeaways: What You Can Expect from QbD 2.0
Traditional QbD misses the behavioral dimension — people, perception, habits, and context
QbD 2.0 adds behavioral science, system psychology, and human-centered design
Start today: Design for decisions, not just for documentation
QbD 2.0 is not a new regulation. It’s a new lens.
A mindset that says: Quality isn’t just what we make — it’s how we think, act, and decide.
Let’s Reflect Together
Have you ever made a decision you weren’t fully sure about — because the system didn’t support another way?
How would your team change if QbD included behavioral design?
Share your thoughts. Let’s build better systems — not just fix broken ones.
Subscribe QMS4 for more real-world insights on quality, audits, and behavioral thinking in GMP.
Want more insights like this?
Connect with me on LinkedIn for weekly tips on GMP, audits, and quality careers.
Subscribe to LinkedIn Newsletter (Quality Career and GMP Insights) | Follow QMS4 | Connect with Lokman